IP High Court Rulings: Pyrimidine Derivative
June 8, 2018
2016 (gyo-ke) No. 10182: Lawsuit seeking Rescission of Trial Decision
2016 (gyo-ke) No. 10184: Lawsuit seeking Rescission of Trial Decision
The IP High Court handed down rulings in the two cases indicated above on April 13, 2018.
The subject patent (Patent No. 2648897) relates to a pyrimidine derivative, and covers Rosuvastatin (HMG-CoA reductase inhibitor), which is a drug for treating hypercholesterolemia. Two invalidation trial against the subject patent were rejected by the Japan Patent Office (JPO), following which the party that had filed the invalidation suits brought both cases before the IP High Court with the aim of having the JPO trial decisions rescinded. The IP High Court rejected both suits.
The points of contention in these cases were the potential benefits to the plaintiff of the lawsuits, inventive step, and the support requirement. In the following, we summarize the rulings on the first two points: the benefits of the suit and inventive step.
■Gist of the ruling regarding the benefits of the suit
It was deemed that there were potential benefits to the plaintiff in the suits for rescission of the trial decisions rejecting the patent invalidation suits, even though the suits were filed after extinction of the patent right. The matter of whether or not the plaintiff is faced with a specific legal disadvantage (whether or not there is an actual dispute regarding compensation for damages or the like or there is the possibility of future dispute) is not a decisive factor in determining the benefits of the suit. However, there are deemed to be no potential benefits in cases in which there is no longer any possibility of compensation for damages or the like (for example, when twenty years have elapsed since the expiration of the patent right).
■Gist of the ruling regarding inventive step
In a case in which a chemical compound is shown in a publication in the form of a chemical formula, and the formula encompasses a vast number of alternative options (more than 20 million options in the present case), in the absence of circumstances that would suggest that a technical concept according to a single specific option should be selected on a proactive or prioritized basis, it is not possible to simply extract a specific technical concept according to the specific option, and the invention described in said publication cannot be recognized as a cited invention that can be compared with the subject invention.
The reason for invalidation of inventive step asserted by the plaintiff was that a practitioner of the art could have easily invented the subject patented invention based on cited reference 1 (Japanese National Phase Publication No. H3-501613), cited reference 2 (Japanese Patent Application Laid-open No. H1-261377), and the technical common knowledge.
The main issue addressed was whether or not, based on the following compound (cited invention 1) disclosed as an HMG-CoA reductase inhibitor in cited reference 1, which was the main reference:
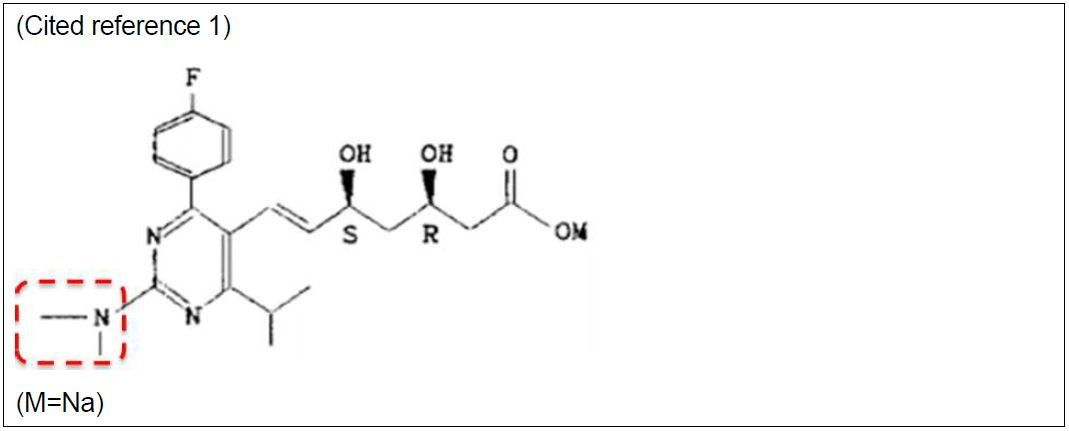
and referring to cited reference 2, a practitioner of the art would have easily arrived at the subject invention:
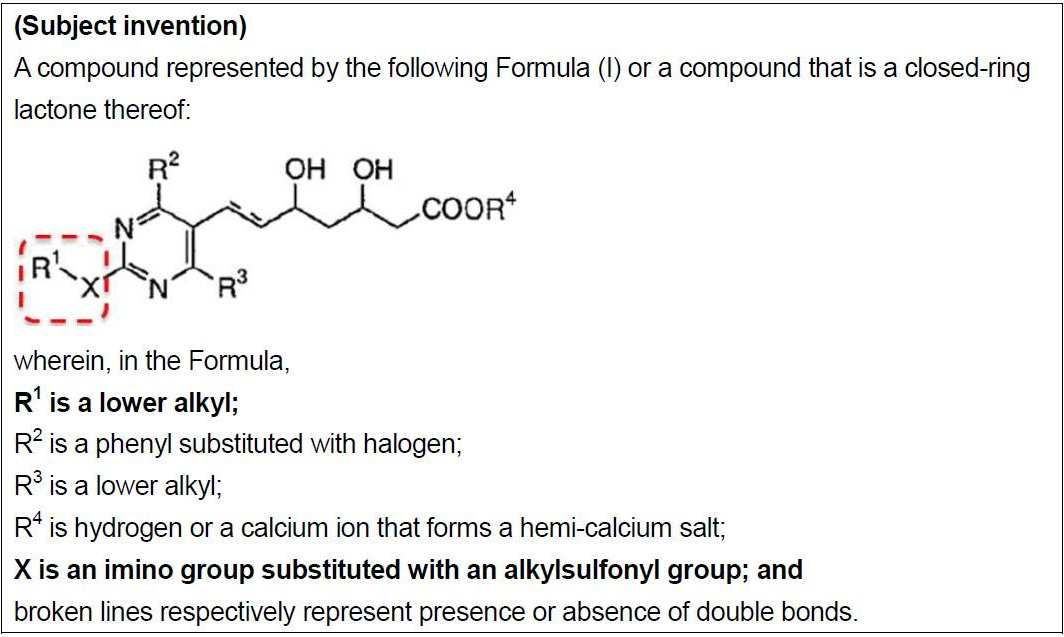
The difference between the subject invention and cited invention 1 is found in the fact that the structure of the substituent groups inside the broken red lines above is -NR1(SO2R') (where R1 is a lower alkyl and R' is an alkyl) in the subject invention and is a dimethyl amino group (-N(CH3)2) in cited invention 1.
The plaintiff asserted that one could easily conceive of the subject invention by substituting the dimethyl amino group of cited invention 1 with -N(CH3)(SO2R') (where R' is an alkyl) based on cited reference 2.
Cited reference 2 discloses the following Formula (I) as an HMG-CoA reductase inhibitor.

Formula (I) of cited reference 2 encompasses cited invention 1. As noted above, a case is included in which R3 in Formula (I) of cited reference 2 is -NR4R5 (where R4 and R5 are an alkyl and an alkylsulfonyl, respectively). Further, the specification describes -NR4R5 as an option for R3 in a "particularly preferable compound", and describes a "methyl group" and an "alkylsulfonyl group" as options for R4 and R5. That is, cited reference 2 discloses a formula that encompasses the -NR1(SO2R') of the subject invention among the range of options thereof. However, the ruling rejected the combination of cited reference 1 and cited reference 2 for mainly the following reasons, and ruled that the inventive step of the subject patented invention is not denied.
(1) The range of options for R3 in the "particularly preferable compound" described in cited reference 2 is extremely large, numbering at least 20 million alternatives.
(2) Cited reference 2 also describes a "particularly extremely preferable compound" in addition to the "particularly preferable compound", and -NR4R5 is not described among the options for R3 in the "particularly extremely preferable compound".
(3) Among the examples of compounds in which X and A in Formula (I) of cited reference 2 have the same configuration as the invention of cited reference 1 (dihydroxyheptenoic acid, which is the active site that is essential for HMG-CoA reductase inhibition), there are no examples in which R3 is -NR4R5.
(4) From the foregoing, there are no circumstances suggesting that -NR4R5 should be selected as R3 in cited reference 2 on a proactive or prioritized basis, and even if -NR4R5 were to be selected, it is unlikely that, in addition, "methyl" and "alkylsulfonyl" would be selected as R4 and R5.
While the rulings also deliberated on the support requirement, the plaintiff's assertions were dismissed and the suits rejected.
These rulings are significant because they provide a framework for considering, in an integrated fashion, the number of alternatives in a formula disclosed in a cited reference together with the motivations for selection of specific options in the cited reference, when evaluating the inventive step of an invention of a compound based on a combination of cited references.
Summary prepared by International Information Group
Please use the contact form below for inquiries or comments.













